Abstract
Background: Current prophylaxis treatments for people with hemophilia A and B can be burdensome, involving frequent intravenous or subcutaneous doses in addition to on demand treatments due to breakthrough bleeds. Fitusiran is an, investigational, subcutaneously administered small interfering RNA therapy, meant to improve thrombin generation, restore hemostasis, and prevent bleeding in all people with hemophilia. Fitusiran has been evaluated in three phase 3 trials: two completed randomized phase 3 clinical trials (ATLAS-INH and ATLAS-A/B), and one phase 3 "switching” study (ATLAS-PPX). Individuals completing any of these 3 parent studies were eligible for the open-label extension study (ATLAS-OLE), which is currently ongoing.
Objective: The aim of this interim analysis is to better understand patients’ and caregivers’ experiences with hemophilia A or B (with or without inhibitors), and its treatments, including fitusiran, and how this experience evolved over the trial period, including their perception and satisfaction with the therapy they received during the trials.
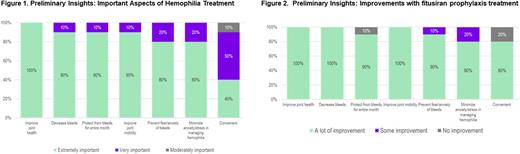
Methods: Patients with hemophilia A or B (PwHA/B) with or without inhibitors, participating in the ATLAS-INH and ATLAS-A/B, and then ATLAS-OLE studies in the United States and India were invited to participate in semi-structured exit interviews to share their experiences. Caregivers of patients in the same studies were invited to participate in the interview if patients were unable (under 18 years of age, cognitively unable). A total of 24 individuals were invited to participate in a 60-minute telephone interview. Following a semi structured interview guide to ensure consistency across the interviews, participants were asked to describe their experiences with hemophilia and its treatment prior to entering any of the ATLAS trials. Next participants were asked about their perceptions of the impact of hemophilia and its treatment on their daily lives, their treatment expectations, and experiences with fitusiran during the ATLAS-OLE trial. Participants were asked to rate different aspects of hemophilia treatments (improving joint health, decreasing bleeds, protection from bleed for entire month, improving joint mobility, prevention of fear/anxiety of bleeds, minimize anxiety/stress in managing hemophilia and convenience) as 'moderately important', 'very important’ or 'extremely important'. Here we presented the data from the interim analysis conducted on the first 10 interviews, analyses of data collected during other interviews are in progress.
Results: For this interim analysis, 8 PwHA/B and 2 caregivers were interviewed, the mean (SD) age was 24.6 (7.4) years. All participants rated improving joint health as extremely important; most participants rated decreasing bleeds (90%), protection from bleeds for an entire month (90%), improving joint mobility (90%), prevention of fear/anxiety of bleeds (80%) and minimize anxiety/stress in managing hemophilia (80%) as 'extremely important’ aspects in their hemophilia treatment (Figure 1). All participants reported improvements in these treatment aspects while under fitusiran prophylaxis with the following percentage of patients having rated "a lot of improvement": 100%, 100%, 90%, 100%, 90%, 80% and 80%, respectively (Figure 2). Majority of participants (n=9) preferred fitusiran prophylaxis over their previous hemophilia treatment and all reported being 'very satisfied’ with fitusiran treatment, especially with regards to its ability to prevent bleeds, durability of effects, and convenience. Among quotes recorded during the interviews, PwHA/B reported for example that "After I took fitusiran, I became like a normal person"; "...very confident because if I get the fitusiran I can say no bleed will happen"; "After I took fitusiran, I became like a normal person"; and "He is more free, He is more confident. That constant threat is not there... generally he is much happier now".
Conclusions: Qualitative insights from this study provides encouraging and positive interim insights into the experiences of PwHA/B, with or without inhibitors, and their caregivers, with fitusiran prophylaxis. Fitusiran treatment demonstrated added value in PwHA/B, irrespective of inhibitor status, particularly resulting in improving joint health, decreasing bleeds, improving joint mobility, and an overall reduction of treatment and disease burden.
Disclosures
Srivastava:Roche: Research Funding; Pfizer: Research Funding; Sanofi: Other: The study was supported by Sanofi, Research Funding; NovoNordisk: Research Funding. Rangarajan:Takeda/Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Reliance Life Sciences: Consultancy, Honoraria, Other: Conference support; Biomarin: Other: Conference Support; Sigilon: Membership on an entity's Board of Directors or advisory committees. Slota:Sanofi: Other: Sanofi contracted RTI Health Solutions to conduct the research study described in the abstract. DiBenedetti:Sanofi: Other: Sanofi contracted RTI Health Solutions to conduct the research study described in the abstract. Cano:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Andersson:Sanofi: Current Employment. Dasmahapatra:Sanofi: Current Employment. Bartelt-Hofer:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Afonso:Sanofi: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal